Why the future of mental health treatment may depend as much on what your client eats as on what they say in session—and the latest research on precision interventions that actually work.
For a century, psychiatry operated on an assumption so fundamental it was rarely questioned: mental illness lives in the brain. Depression is neurotransmitter imbalance. Anxiety is an overactive amygdala. Trauma is encoded in neural circuits. The body below the neck was largely irrelevant—a vehicle for transporting the troubled brain to the therapist’s office.
That assumption is collapsing under the weight of evidence.
The research emerging from 2024 and 2025 doesn’t merely suggest that nutrition affects mental health—that’s been known for decades. It demonstrates mechanisms. It identifies specific strains of bacteria that reduce cortisol. It establishes threshold doses of omega-3s that match antidepressant efficacy. It issues safety warnings about previously “safe” herbs causing liver damage. We are no longer in the realm of wellness advice. We are entering precision nutritional psychiatry—where interventions are targeted to specific biological profiles with the same rigor we apply to pharmaceutical prescribing.
For psychotherapists, this research doesn’t replace what we do. It explains why some clients remain stuck despite excellent therapeutic work. It offers tools for the body-brain connection we’ve always intuited but couldn’t quite articulate. And it validates what somatic and experiential approaches have long maintained: you cannot heal the mind while ignoring the body that houses it.
The Gut-Brain Revolution Gets Specific
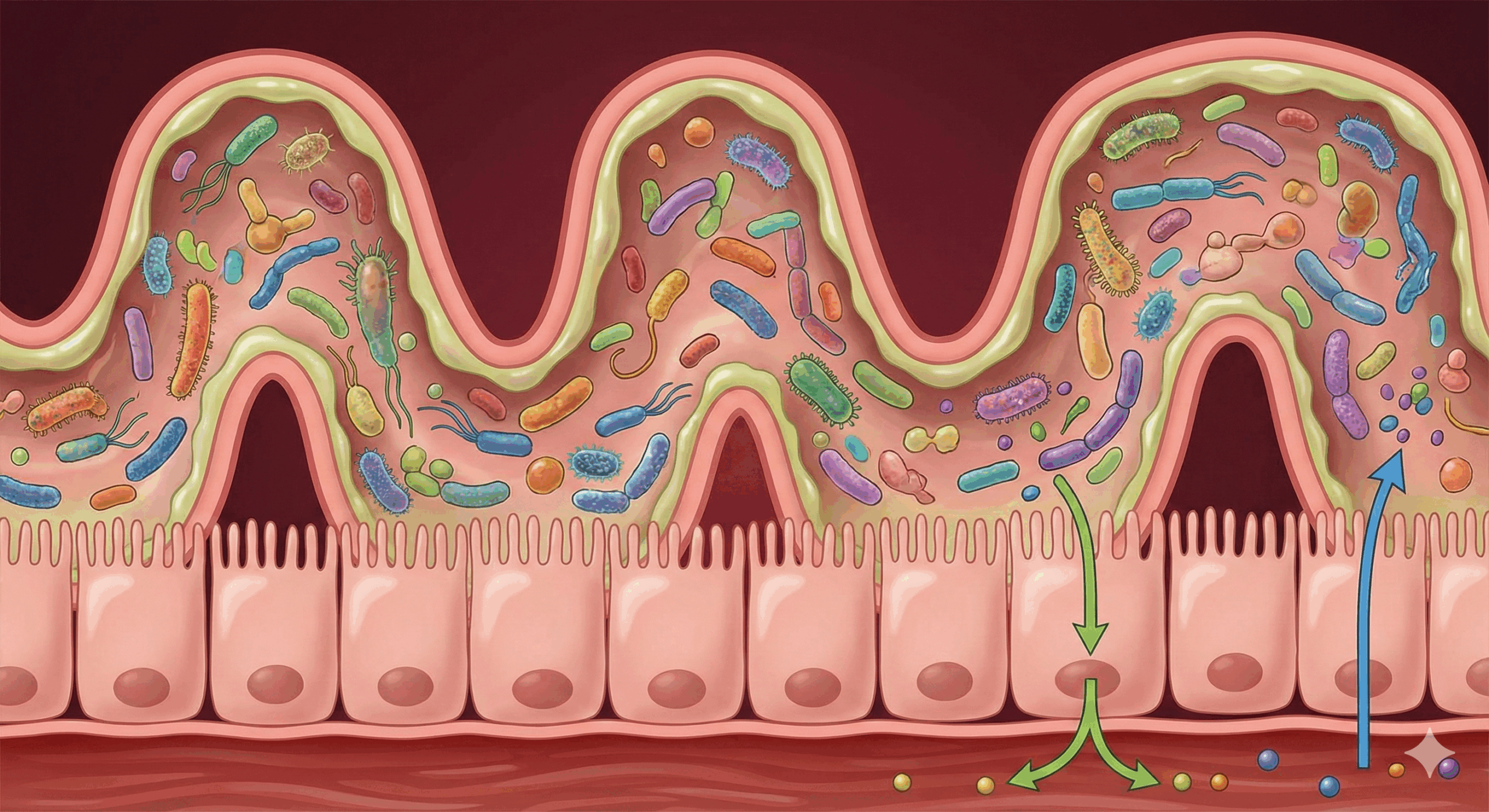
The gut-brain axis has been the subject of excited speculation for years. But 2024-2025 research has moved decisively from “gut bacteria affect mood” to identifying exactly which bacteria, through which mechanisms, for which conditions.
The brain-gut connection, as Johns Hopkins Medicine describes it, operates through multiple simultaneous pathways. The vagus nerve carries signals directly from gut to brainstem. Microbial metabolites cross the blood-brain barrier. Immune mediators triggered by gut inflammation activate microglia—the brain’s immune cells—which then “prune” synaptic connections and reduce serotonin availability.
This isn’t metaphor. When the gut lining is compromised by stress, processed food, or alcohol, bacterial lipopolysaccharides leak into the bloodstream and trigger systemic inflammation. That inflammation crosses into the brain. The result is measurable: elevated inflammatory markers in depressed patients correlate with reduced hippocampal volume and impaired neuroplasticity. You cannot think your way out of neuroinflammation. You cannot process trauma effectively when your brain is metabolically compromised.
The term “psychobiotics” now refers to specific bacterial strains with demonstrated mental health effects. And the research has become remarkably precise. A 2024 meta-analysis confirmed that efficacy is entirely strain-dependent—generic “probiotic” supplements are essentially useless for mental health. What works are specific combinations: Lactobacillus helveticus R0052 paired with Bifidobacterium longum R0175 consistently reduces cortisol and alleviates physical symptoms of stress by dampening HPA-axis reactivity. Lactobacillus plantarum P8 reduces anxiety through anti-inflammatory pathways. Bifidobacterium breve improves both mood and sleep by modulating circadian-relevant metabolites.
A critical nuance emerged from the 2024 data: psychobiotics appear significantly more effective as adjuncts to antidepressants than as monotherapy for severe depression. For mild-to-moderate cases, they may serve as standalone interventions. But in clinical populations, they function best as “biological primers”—optimizing the physiological environment so that other treatments can work. This is precisely how we should think about them in psychotherapy: not replacements for therapeutic work, but biological foundations that make that work possible.
Parallel research from the University of Virginia School of Medicine identified that Lactobacillus strains found in fermented foods help maintain interferon-gamma, an immune mediator regulating stress response. Unlike isolated probiotic supplements, fermented foods provide a complex matrix—the bacteria plus prebiotics plus fermentation byproducts—that creates more resilient gut ecosystems than single-strain capsules. For clients resistant to supplements, “a daily serving of living food”—kefir, kimchi, sauerkraut, yogurt—is an evidence-based intervention addressing biological roots of distress while fostering healthier relationships with nutrition.
The Foundational Four: What the Latest Data Actually Shows
Novel compounds grab headlines, but the “Big Four” nutrients—Vitamin D, Magnesium, Zinc, and Omega-3s—remain the bedrock of nutritional psychiatry. The 2024-2025 research hasn’t just reinforced their importance; it has provided critical clarity on dosing, forms, and target populations.
Vitamin D is often mischaracterized as merely a bone nutrient. In reality, it functions as a neurosteroid hormone with receptors throughout the brain, particularly in the hippocampus and prefrontal cortex, regulating serotonin and dopamine synthesis and modulating BDNF (Brain-Derived Neurotrophic Factor). A 2025 meta-analysis of twenty randomized controlled trials definitively concluded that Vitamin D supplementation significantly reduces depressive symptoms—but with crucial caveats. The benefit is most pronounced in individuals with baseline deficiency, in those with diagnosed Major Depressive Disorder (where effect sizes nearly double), and in patients with medical comorbidities. Supplementing people with adequate levels yields diminishing returns.
A 2024 analysis distinguished between D2 and D3, finding that serum D3 (cholecalciferol) was strongly associated with reduced depression risk while D2 (ergocalciferol) showed weaker associations. The Endocrine Society’s 2024 guidelines suggest that high-risk groups often need doses above standard RDA to reach therapeutic serum levels. For psychiatric sufficiency, clinicians should aim for the upper end of optimal range (40-60 ng/mL).
Magnesium’s role as a natural NMDA receptor antagonist and GABA agonist makes it pivotal for anxiety and stress regulation—it acts as the nervous system’s “brake,” counteracting excitatory glutamate. This aligns with physiological regulation strategies we use in interventions for panic and dissociation. A 2024 systematic review found significant improvements in self-reported anxiety with magnesium supplementation, though sleep evidence remains mixed—likely because trials used different forms. Magnesium glycinate provides glycine (an inhibitory neurotransmitter aiding sleep), while L-threonate demonstrates unique ability to cross the blood-brain barrier in preclinical models. For clients presenting with “tired but wired” anxiety, muscle tension, and insomnia, magnesium remains a high-yield, low-risk intervention addressing physiological hyperarousal that often impedes progress in somatic therapies.
Zinc is a cofactor for over 300 enzymes, playing critical roles in synaptic plasticity and BDNF regulation. Zinc deficiency mimics depression: lethargy, anhedonia, cognitive slowing. A recent meta-analysis solidified zinc’s role as an augmentation strategy—when added to SSRI therapy at 25-30mg daily, it significantly enhances antidepressant response and reduces latency to onset. A 2024 study in older adults demonstrated that zinc supplementation alone reduced depression and anxiety scores over 70 days, accompanied by rising serum BDNF. The mechanism involves NMDA receptor inhibition (similar to ketamine, though weaker) and hippocampal BDNF expression—making zinc particularly indicated for treatment-resistant depression where standard monoaminergic drugs have failed.
Omega-3 fatty acids regulate neuronal membrane fluidity and suppress neuroinflammation. The “EPA vs. DHA” debate has largely been settled: formulations must contain high ratios of eicosapentaenoic acid (EPA) to be effective for mood. The 2024-2025 consensus from the International Society for Nutritional Psychiatry Research is clear: doses exceeding 1g/day of EPA show significant antidepressant effects, particularly in patients with elevated inflammatory markers. Research from Massachusetts General Hospital found that high-dose omega-3s improved cognition specifically in depressed patients with elevated inflammation—suggesting these fats work partly by addressing the “inflammatory subtype” of depression.
New 2024 research involving military personnel demonstrated that high omega-3/low omega-6 diets significantly reduced post-traumatic headaches and pain intensity. And a meta-analysis of 28 RCTs found omega-3 supplementation reduced aggression by 22% across diverse populations—from children to incarcerated adults—with profound implications for treating conduct disorder and antisocial traits.
When recommending fish oil for mood, “counting grams of fish oil” is useless. Clinicians must teach clients to read labels for EPA content. The evidence-based therapeutic window for depression is 1000-2000mg of EPA daily.
Amino Acid Therapies: Precision Tools for Neurochemistry
Amino acids are neurotransmitter precursors, and targeted amino acid therapy offers precision medicine approaches often overlooked in favor of vitamins. These compounds address specific pathways with specificity approaching pharmaceuticals.
N-Acetylcysteine (NAC) may be the most versatile supplement in the psychiatric pharmacopeia. As a precursor to glutathione (the body’s master antioxidant) and a modulator of glutamate transmission, it targets two core pathologies: oxidative stress and excitotoxicity. A 2024 systematic review confirmed NAC’s efficacy as an adjunct for moderate-to-severe OCD, showing beneficial trends on Y-BOCS scores. Its ability to dampen habitual and compulsive neural loops makes it a prime candidate for trichotillomania, excoriation disorder, and substance use disorders—conditions where the “urge” component drives behavior. By restoring glutamate homeostasis in the nucleus accumbens, NAC reduces craving without sedation or cognitive impairment.
L-Theanine, found in green tea, is structurally similar to glutamate but acts as a calming agent. 2025 EEG research confirms that L-theanine supplementation increases alpha brain wave activity (8-12 Hz)—the signature of “relaxed alertness” similar to meditation. This distinguishes it from sedatives that induce drowsiness through theta/delta waves. A 2025 systematic review of 13 trials found that 200-450mg significantly improved sleep quality and reduced subjective stress without cognitive impairment—making it an ideal “daytime anxiolytic” for clients who need to remain functional at work.
Taurine, often dismissed as an energy drink ingredient, is actually a potent cytoprotective amino acid. A major 2024 study involving 12,000 adults found higher taurine levels correlated with lower obesity, reduced inflammation, and lower type 2 diabetes rates. In animal models, it extended lifespan and reduced depression-like behaviors. Taurine acts as a GABA receptor agonist while protecting neurons from excitotoxicity and mitochondrial dysfunction. While human trials on cognition are still emerging, its safety profile makes it worth monitoring.
Creatine plays a crucial role in brain bioenergetics—the brain consumes 20% of the body’s energy despite being 2% of body weight. A 2025 study demonstrated that 5g of creatine daily, when added to CBT, enhanced treatment outcomes for depression. However, a significant caveat remains for bipolar disorder: creatine can induce manic switches in susceptible individuals due to its potent enhancement of ATP availability and dopaminergic function. It should be used with extreme caution—or avoided entirely—in this population.
Herbal Interventions: Promise and Peril
The herbal psychopharmacology landscape has seen both exciting validation and sobering safety signals in 2024-2025.
Ashwagandha (Withania somnifera) has exploded in popularity for its ability to lower cortisol and improve sleep. 2025 systematic reviews continue to support its efficacy in significantly reducing serum cortisol, improving sleep quality, and reducing anxiety scores. But a critical development demands attention: the emergence of liver toxicity cases associated with ashwagandha. Several European health agencies—Denmark, France, the UK—have issued warnings or bans following case reports of cholestatic liver injury. The NIH Office of Dietary Supplements notes these concerns while acknowledging the cases are rare.
This is a crucial lesson: “natural” does not mean safe. The “more is better” approach to adaptogens is dangerous. Clinicians must advise clients to cycle this herb rather than taking it indefinitely, and to monitor for signs of jaundice or unusual fatigue—especially with pre-existing liver conditions.
Saffron (Crocus sativus) has emerged as the most compelling herbal antidepressant. Multiple 2024 meta-analyses have found saffron extract non-inferior to SSRIs for treating mild-to-moderate depression—with a significantly better side effect profile, particularly regarding sexual dysfunction. Its active constituents, crocin and safranal, appear to inhibit reuptake of serotonin, dopamine, and norepinephrine while providing antioxidant protection and supporting neurogenesis. A comprehensive review confirmed these mechanisms. For clients concerned about SSRI side effects but needing pharmacological support, saffron represents a legitimate evidence-based alternative for mild-to-moderate presentations.
Rhodiola rosea shows specific utility for burnout and mental fatigue. Unlike caffeine, which borrows energy, rhodiola appears to optimize mitochondrial efficiency. 2025 data showed it improved endurance and cognitive processing speed under fatigued conditions—making it particularly relevant for the exhausted, depleted clients who increasingly present in our practices.
Curcumin, the active compound in turmeric, is primarily an anti-inflammatory. Since approximately 30-40% of depression appears driven by inflammation (the “inflammatory subtype”), curcumin is specifically indicated for these patients. However, 2024 research reinforces that standard turmeric powder is ineffective for brain health due to poor absorption. Specialized formulations—phytosome or piperine-enhanced—are required to cross the blood-brain barrier.
Broad-Spectrum Micronutrients: The Orchestra, Not the Soloist
Perhaps the most paradigm-shifting research comes from broad-spectrum micronutrient (BSM) therapy. Unlike the “magic bullet” approach of single-nutrient trials, BSM posits that the brain needs a full orchestra of nutrients to function—not just one soloist playing louder.
The MADDY Study (Micronutrients for ADHD in Youth) and its 2024-2025 follow-ups demonstrated that a specific multi-nutrient formula significantly improves emotional regulation, aggression, and global functioning in children with ADHD. The key insight: responders were often those with high irritability and emotional lability—symptoms notoriously difficult to treat with stimulants alone. This research is particularly relevant given how often emotional dysregulation, not attention per se, drives impairment in ADHD populations.
The NUTRIMUM trial (2024) investigated BSM for antenatal depression. Pregnant women taking broad-spectrum micronutrients had significantly greater improvements in global functioning and sleep compared to placebo, with no adverse effects on infants. This offers a vital alternative for pregnant women hesitant to take psychopharmaceuticals—a population where untreated depression carries significant risks but medication concerns are legitimate.
The emerging research on BSM for emotional dysregulation suggests these formulas may work by addressing the cumulative effects of marginal deficiencies—no single nutrient is dramatically low, but many are suboptimal, and the combined effect compromises brain function. This resonates with what we see clinically: clients eating “okay” diets that nonetheless leave their nervous systems unsupported.
Clinical Implications: What This Means for Psychotherapy
This research is not a mandate for therapists to prescribe supplements. It is a call to integrate biological awareness into our clinical thinking—and to recognize when biological factors may be limiting therapeutic progress.
When a client remains stuck despite insight and intervention, the question “What are you eating?” is not a distraction from psychological work. It is an investigation into their capacity for neuroplasticity. A client running on processed food, deficient in omega-3s, with a depleted microbiome may literally lack the biological substrate for the neural changes therapy requires. Their “resistance” may be metabolic, not psychological.
We can offer psychoeducation that reduces shame. Explaining that chronic inflammation is a driver of their depression reframes the problem from “character flaw” to “biological state”—and opens pathways for intervention that don’t require them to simply “try harder” at thinking differently. This aligns with how we understand trauma as a physiological phenomenon, not merely a cognitive one.
We can make targeted referrals. Identifying signs of nutritional deficiency—poor night vision (zinc), brittle nails (iron), “tired but wired” presentation (magnesium), chronic soft-tissue pain (vitamin D)—allows for referral to functional medicine practitioners or psychiatrists who understand nutritional biochemistry. Vitamin D levels, inflammatory markers, and microbiome testing are increasingly accessible and clinically relevant.
We can support lifestyle interventions that address biological roots of distress. Recommending fermented foods, omega-3 rich fish, or colorful vegetables isn’t wellness fluff—it’s evidence-based intervention for the gut-brain connection that underlies mood regulation.
And we must emphasize safety. The 2024 liver injury reports regarding ashwagandha remind us that natural does not mean harmless. Clients should inform prescribing physicians of all supplements (St. John’s Wort, for example, interacts dangerously with SSRIs and many other medications). Adaptogens should be cycled rather than taken indefinitely. High-quality, third-party tested brands reduce contamination risks. And any supplement causing unusual symptoms—fatigue, jaundice, digestive disturbance—should be stopped and evaluated.
The Convergence of Psyche and Soma
The research of 2024-2025 has irrevocably bridged the gap between plate and psyche. We are moving toward precision nutritional psychiatry, where interventions aren’t generic “eat healthy” advice but targeted protocols: this strain of Lactobacillus for your HPA-axis dysregulation, this ratio of EPA for your inflammatory depression, this form of magnesium for your glutamate-driven anxiety.
For psychotherapists, this represents an expansion of our toolkit, not a replacement of our skills. The relationship remains central. Meaning-making remains essential. Trauma still needs processing, patterns still need understanding, and attachment still matters. But we now understand that the biological environment of the brain—its inflammation levels, neurotransmitter precursor availability, mitochondrial energy production, and microbial ecosystem—is the soil from which psychological resilience or pathology grows.
Depleted soil grows stunted plants no matter how much you encourage them. Healthy soil supports growth almost effortlessly.
The message for our clients is ultimately empowering: mental health is not just “in your head.” It is in your gut, your mitochondria, your cell membranes, your inflammatory markers. And you have more tools to support it than ever before. Not instead of therapy. Not instead of medication when needed. But alongside them—creating the biological conditions in which healing can actually take root.
The body keeps the score in more ways than we knew. And attending to that body—feeding it, nourishing its microbial partners, providing the raw materials its neurons require—may be as essential to mental health as anything we say in the therapy room.
References and Further Reading
Gut-Brain Axis and Psychobiotics:
Johns Hopkins Medicine: The Brain-Gut Connection
Cleveland Clinic: Benefits of Fermented Food for Mental Health
UVA Health: How Fermented-Food Bacteria Guard Against Depression
PubMed: Strain-Specific Effects of Probiotics on Depression and Anxiety
Foundational Nutrients:
PubMed: Meta-Analysis of Vitamin D Effect on Depression
Endocrine Society: 2024 Vitamin D Guidelines
PMC: Effects of Magnesium on Anxiety and Sleep
Open Public Health Journal: Zinc Supplementation in Elderly Depression
MGH Psychiatry: High-Dose Omega-3s in Inflammatory Depression
PMC: Omega-3 Supplementation Reduces Aggression
Amino Acid Therapies:
PMC: NAC as Augmentation for OCD
PubMed: L-Theanine Effects on Sleep
Columbia University: Taurine and Longevity
PMC: Creatine Supplementation in Depression
Herbal Interventions:
NIH Office of Dietary Supplements: Ashwagandha
NIH LiverTox: Ashwagandha Hepatotoxicity
PubMed: Saffron vs. SSRIs Meta-Analysis
Psychiatry Redefined: Curcumin in Functional Psychiatry
Broad-Spectrum Micronutrients:
PubMed: NUTRIMUM Trial for Antenatal Depression
PMC: Broad-Spectrum Micronutrients for Emotional Dysregulation



















0 Comments