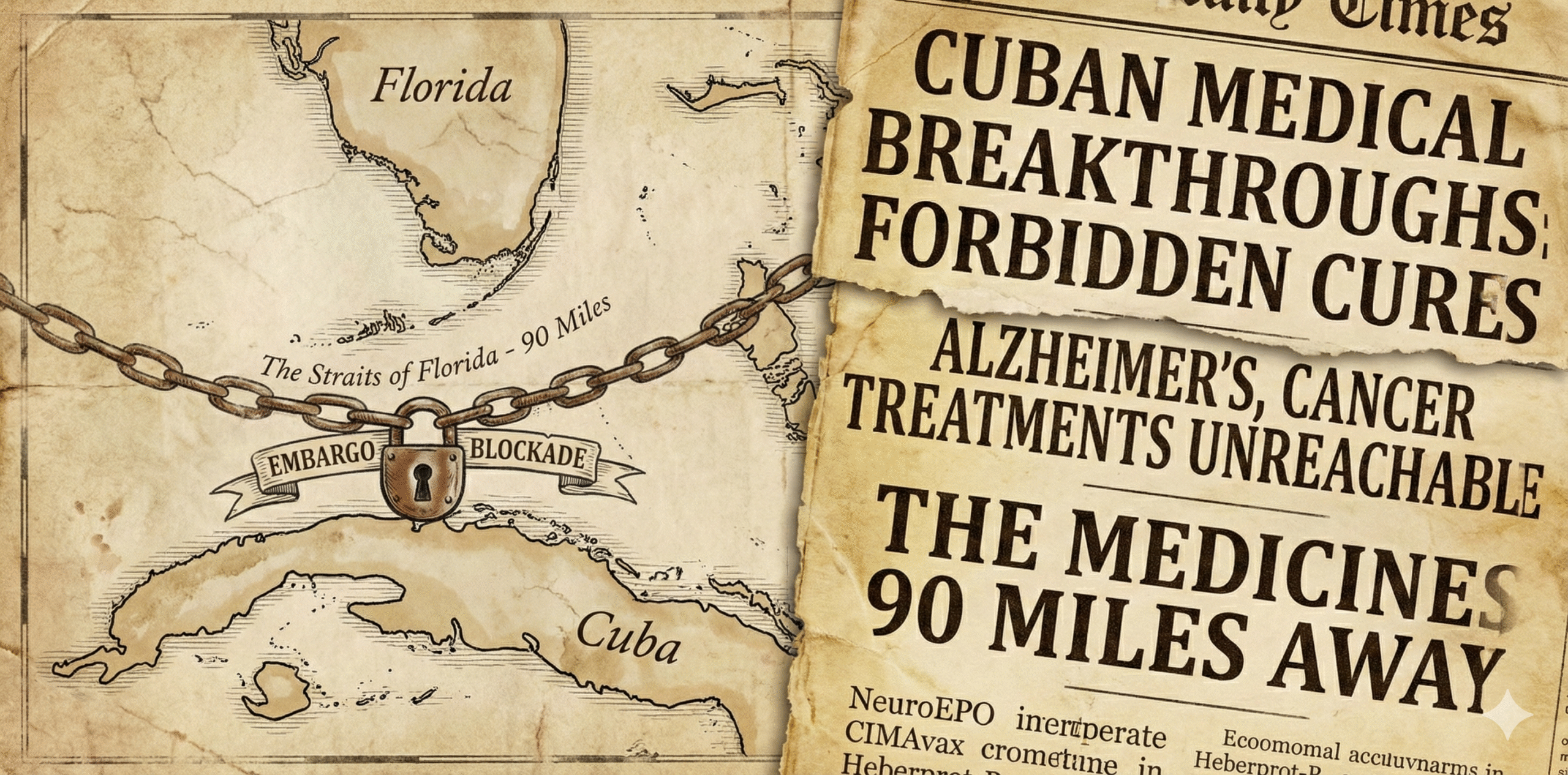
The 90-Mile Medicine Gap:
Inside the paradox of American pharmaceutical innovation: We lead the world in drug development but struggle to translate that into affordable, accessible care—while a small island nation under economic embargo may have developed better solutions we’re legally blocked from using.
There’s an Alzheimer’s drug being used in Cuba right now that shows a 7-point improvement on cognitive assessment scales in clinical trials. The newest FDA-approved Alzheimer’s drug in the United States shows roughly a 1.4-point difference. The Cuban drug is a nasal spray you use at home. The American drug requires biweekly IV infusions at a hospital, costs over $26,000 per year before monitoring costs, and carries a risk of brain bleeding that requires regular MRI surveillance.
Americans can’t legally access the Cuban drug.
This isn’t an isolated case. Cuba has developed a lung cancer vaccine that has kept terminal patients alive for years beyond their prognosis—available free to Cuban citizens since 2011. They’ve developed a diabetic wound treatment that reduces amputation risk by over 70%. Both are effectively off-limits to Americans due to a 60-year trade embargo.
But here’s what makes this story more than just a tale of geopolitical absurdity: even if the embargo ended tomorrow, American pharmaceutical companies would likely have no interest in developing or distributing these drugs. The economics don’t work. They’re too cheap. They cure too quickly. They can’t be patented for monopoly pricing.
This article examines three Cuban medical innovations, compares them to American standards of care, and explores the structural reasons why the world’s most expensive healthcare system consistently fails to produce the most accessible or effective treatments. It’s a story about science, money, politics, and the uncomfortable question of what “innovation” actually means when patients can’t afford or access the results.
Part One: The Alzheimer’s Divide
What We’re Working With in America
Alzheimer’s disease affects more than 7 million Americans, with that number projected to nearly double by 2050. For decades, the treatment landscape was bleak—we had drugs that temporarily improved symptoms but did nothing to slow the underlying disease progression. Then, in 2021 and 2023, the FDA approved two new medications that promised to change everything: aducanumab (Aduhelm) and lecanemab (Leqembi).
These drugs represent the culmination of the “amyloid hypothesis”—the dominant theory in Alzheimer’s research for over 30 years. The idea is straightforward: Alzheimer’s brains accumulate sticky protein clumps called amyloid plaques. Remove the plaques, stop the disease. Both drugs are sophisticated biological weapons designed to do exactly that. They’re monoclonal antibodies—laboratory-engineered proteins that bind to amyloid and tag it for removal by the brain’s immune cells.
The problem is that removing plaques hasn’t translated into dramatic clinical improvement.
The pivotal trial for Leqembi (called CLARITY AD) enrolled nearly 1,800 patients with early Alzheimer’s. After 18 months, patients on the drug showed a 27% slowing of cognitive decline compared to placebo. That sounds meaningful until you look at the actual numbers: a difference of 0.45 points on an 18-point scale. Researchers debate whether patients or families can even perceive a difference that small.
Meanwhile, the safety concerns are serious. Because amyloid doesn’t just accumulate in brain tissue—it also builds up in the walls of blood vessels—removing it can destabilize those vessels. The result is a phenomenon called ARIA (Amyloid-Related Imaging Abnormalities): brain swelling in about 13% of patients, brain bleeding in about 17%. Most cases are asymptomatic and resolve, but some are severe. There have been deaths, particularly in patients taking blood thinners.
This safety profile requires extensive monitoring. Patients need MRI scans before starting treatment and multiple follow-up scans during the first year. They need to receive biweekly IV infusions at specialized medical centers. The drug itself costs $26,500 per year, but total treatment costs—including infusions, monitoring, and radiologist fees—can exceed $90,000 annually.
And here’s the critical point: even with all this expense and risk, the drug doesn’t stop the disease. It slows it modestly. Patients still decline. They just decline slightly slower than they would have otherwise.
What Cuba Developed Instead
While American researchers pursued the amyloid hypothesis with billions in funding, Cuban scientists at the Center for Molecular Immunology took a different approach. They weren’t trying to remove plaques. They were trying to protect neurons from dying in the first place.
Their drug is called NeuroEPO (marketed as NeuralCIM). It’s based on erythropoietin—a hormone your body naturally produces that’s best known for stimulating red blood cell production. Athletes have abused synthetic EPO for decades to boost their oxygen-carrying capacity. But researchers discovered that EPO also has powerful effects in the brain: it reduces inflammation, protects neurons from oxidative stress, and inhibits programmed cell death.
The problem with using standard EPO for brain conditions is that the doses needed to reach the brain would massively overstimulate blood cell production, thickening the blood and causing strokes. The Cuban solution was elegant: they engineered a version of EPO with low sialic acid content. This modification means the drug gets rapidly cleared from the bloodstream before it can affect blood cells—but if you deliver it directly to the brain via nasal spray, it reaches therapeutic concentrations in the central nervous system without systemic effects.
The clinical trial results were striking. In the ATHENEA trial, 174 patients with mild-to-moderate Alzheimer’s received either NeuroEPO or placebo for 48 weeks. The primary outcome measure was the ADAS-Cog11, a standard cognitive assessment tool where higher scores mean worse impairment.
The placebo group got worse, as expected—their scores increased by about 4 points. The NeuroEPO group actually improved, with scores decreasing by 3-4 points. The net difference between groups was 7 points.
For context: approved Alzheimer’s drugs like donepezil typically show 2-3 point differences. Leqembi showed about 1.4 points on a comparable scale. A 7-point swing is, by the standards of Alzheimer’s research, enormous.
Additionally, 80% of patients in the treatment group showed either no decline or actual clinical improvement. Brain perfusion imaging showed improved blood flow in the brain regions most affected by Alzheimer’s. And the safety profile was benign—mostly mild nasal irritation, with no brain bleeding, no need for MRI monitoring, and stable blood counts confirming the modified EPO wasn’t affecting blood cell production.
The Comparison That Should Trouble Us
Let’s put this side by side:
Leqembi (USA): IV infusion every two weeks at a medical center. Risk of brain swelling (13%) and bleeding (17%). Requires multiple MRI scans. Annual cost exceeding $90,000 with monitoring. Clinical effect: slows decline by 27%, roughly 0.45 points on rating scales.
NeuroEPO (Cuba): Nasal spray three times weekly at home. No serious adverse events. No special monitoring required. Priced for universal access in Cuba’s public health system. Clinical effect: 7-point improvement versus placebo, with 80% of patients stabilizing or improving.
Now, important caveats: these were different trials with different patient populations, different outcome measures, and different durations. Cross-trial comparisons are inherently limited. The Cuban trial was smaller and hasn’t been replicated in Western settings yet—though a Phase 2 trial at the University of Saskatchewan is currently underway to validate the findings using North American standards.
But even accounting for these limitations, the disparity is hard to ignore. One approach removes plaques with significant risks and modest benefits. The other protects neurons with minimal risks and potentially larger benefits. One requires hospital infrastructure and costs as much as a new car annually. The other can be used at home and was designed for a resource-limited public health system.
Why didn’t American researchers develop something like NeuroEPO? The answer lies in how scientific research gets funded in the United States.
Part Two: The Lung Cancer Paradox
The U.S. Standard: Checkpoint Inhibitors
Lung cancer kills more Americans than any other cancer—about 127,000 people per year. The past decade has seen a revolution in treatment with the advent of immune checkpoint inhibitors like Keytruda (pembrolizumab) and Opdivo (nivolumab). These drugs work by releasing the “brakes” on the immune system, allowing T-cells to attack tumors they would otherwise ignore.
For patients whose tumors express high levels of a protein called PD-L1, these drugs have been transformative. Five-year survival rates for advanced lung cancer have improved from nearly zero to 20-25% in some populations. That’s genuine progress.
But checkpoint inhibitors have significant limitations. They don’t work well for patients with low PD-L1 expression—a substantial portion of lung cancer patients. They can cause serious autoimmune side effects as the unleashed immune system attacks healthy tissues: colitis, pneumonitis, thyroid dysfunction. And they cost over $150,000 per year.
Cuba’s Alternative: Starving the Tumor
Cuba’s approach to lung cancer took a completely different path. Rather than unleashing the immune system to attack cancer, Cuban researchers developed CIMAvax-EGF—a therapeutic vaccine that trains the body to attack something the cancer needs to survive.
Most lung cancers depend heavily on a growth signal called Epidermal Growth Factor (EGF). This protein normally helps cells grow and divide, but cancer cells hijack it for their own proliferation. American pharmaceutical companies have developed drugs that block the EGF receptor on cancer cells—drugs like Tagrisso. But tumors frequently develop resistance mutations that let them ignore these blockers.
CIMAvax takes a different approach. Instead of blocking the receptor, it teaches the immune system to attack the EGF molecule itself. The vaccine combines recombinant human EGF with a carrier protein and adjuvant. When injected, it stimulates the production of antibodies that bind to circulating EGF, preventing it from reaching the tumor. You’re essentially starving the cancer of its food supply.
Because the target is the body’s own EGF (which remains stable) rather than the tumor itself (which mutates rapidly), resistance is far less likely to develop.
Clinical trial data from Cuba showed significant benefits. In patients with high baseline EGF levels, median overall survival extended to 14.66 months compared to 8.63 months in controls—a 70% improvement. The five-year survival rate for vaccinated patients was 23%; for controls, it was 0%.
The vaccine has been available free to Cuban citizens since 2011 and is now approved in several other countries including Colombia, Peru, and Paraguay.
The American Exception: Roswell Park
There is one U.S. institution that has received special permission to study CIMAvax: Roswell Park Comprehensive Cancer Center in Buffalo, New York. Their research has revealed something particularly interesting.
When CIMAvax is combined with Opdivo, it appears to help patients who wouldn’t normally respond to checkpoint inhibitors alone. Specifically, patients with low PD-L1 expression—the “non-responders” to standard immunotherapy—showed promising responses to the combination. The three-year overall survival rate in the study was 29%, with the combination appearing to “wake up” cold tumors that checkpoint inhibitors alone couldn’t activate.
The safety profile was notably mild compared to standard immunotherapy—mostly injection site reactions and low-grade fever, without the serious autoimmune complications of checkpoint inhibitors.
Yet despite these promising results, CIMAvax remains unavailable to most American patients. The regulatory pathway from Cuban clinical trials to FDA approval is effectively blocked by the embargo’s licensing restrictions. Roswell Park can study the drug, but the path to commercial availability remains uncertain.
The Cost Comparison
CIMAvax costs approximately $1 to produce per dose. Even with American markups, it would represent a fraction of the $150,000+ annual cost of checkpoint inhibitor therapy. It doesn’t require the intensive monitoring or management of autoimmune side effects that Keytruda and Opdivo demand.
From a pure cost-effectiveness standpoint, it’s hard to imagine a better candidate for development. But American pharmaceutical economics don’t reward cost-effectiveness—they reward high margins and market exclusivity.
Part Three: The Amputation Crisis
A Uniquely American Tragedy
Every year, approximately 73,000 Americans with diabetes undergo amputations due to foot ulcers that won’t heal. That’s one amputation every seven minutes. Half of those patients will die within five years of losing their limb—a mortality rate higher than most cancers.
These amputations are not evenly distributed. Black Americans are 2-4 times more likely to undergo diabetic amputation than white Americans. Native Americans face rates nearly double those of white populations. Medical researchers have called diabetic amputations a “mega-disparity”—one of the starkest examples of healthcare inequality in America.
The standard treatment pathway in the U.S. involves wound care: debridement (scraping away dead tissue), specialized dressings, pressure-relieving boots, and in some cases hyperbaric oxygen therapy. There’s one FDA-approved topical growth factor—Regranex (becaplermin)—but it shows only modest efficacy and carries a black box warning for increased cancer mortality.
The problem is that chronic diabetic wounds create a “hostile soil” for healing. The wound bed is filled with enzymes (proteases) that destroy growth factors before they can penetrate deep enough to stimulate repair. Topical treatments sit on the surface, unable to reach the cells that need to regenerate.
Cuba’s Solution: Going Deep
Heberprot-P, developed by Cuba’s Center for Genetic Engineering and Biotechnology, takes a fundamentally different approach. Rather than applying growth factors to the wound surface, it injects recombinant human Epidermal Growth Factor directly into the wound bed and edges—deep into the tissue where the healing needs to happen.
This intralesional delivery bypasses the proteases on the wound surface and delivers high concentrations of EGF directly to the fibroblasts and epithelial cells that need to proliferate. The effect is described as “waking up” cells that have become senescent in the hostile diabetic wound environment, triggering rapid granulation (new tissue formation) from the bottom up.
The clinical results have been remarkable. In controlled trials and real-world use spanning over 290,000 patients worldwide, Heberprot-P has consistently reduced the relative risk of amputation by more than 70%. In patients with deep, grade 3-4 ulcers (including some with gangrene), 77% achieved complete wound granulation compared to 56% with standard care.
The drug has been registered in Cuba since 2006 and is now available in over 20 countries. The National Institutes of Health has acknowledged it as a “novel product that provides an opportunity to address an important unmet medical need.”
Yet it’s not available in the United States. And the story of why reveals everything broken about American pharmaceutical development.
The Licensing Catch-22
In 2016, researchers at the University of Arizona began discussions with Cuban developers to bring Heberprot-P through FDA clinical trials. They had the science. They had the interest. What they didn’t have was a clear path to commercialization.
The Treasury Department’s Office of Foreign Assets Control (OFAC) regulates transactions with Cuba under the embargo. OFAC approved the clinical trial itself. But they wouldn’t commit to granting a license for commercial sale even if the FDA approved the drug.
Without that guarantee, no private investor would put up the $100+ million required for a Phase III trial. The risk was asymmetric: spend enormous sums on research, potentially prove the drug works, and then have no legal pathway to sell it. The University of Arizona project stalled. The drug that could prevent tens of thousands of American amputations remains 90 miles and a world away.
Part Four: Why American Pharma Won’t Fill the Gap
You might think that if Cuban drugs are genuinely effective, American pharmaceutical companies would simply develop their own versions. The molecules aren’t magic—EGF and EPO are well-characterized proteins. Why not engineer American alternatives?
The answer reveals the structural dysfunction at the heart of American pharmaceutical economics.
The Patent Problem
The U.S. pharmaceutical business model depends on patent exclusivity. A company spends hundreds of millions (or billions) on drug development with the expectation of 15-20 years of monopoly pricing to recoup that investment. This model favors Novel Chemical Entities (NCEs)—new molecules that can be patented from scratch.
EGF and EPO aren’t new. They’ve been known and characterized for decades. While Cuba has specific formulation patents (for the low-sialic acid EPO, for the intralesional EGF delivery method), the underlying molecules are off-patent. An American company developing similar drugs would face immediate generic competition or would need to license Cuban intellectual property—which OFAC makes nearly impossible.
Even if licensing were possible, the market exclusivity window would be short. Cuban formulation patents are already aging. A company that spent five years on FDA trials might have only a few years of monopoly pricing before generics could enter. That math doesn’t work for American investors seeking blockbuster returns.
The Cure vs. Treatment Problem
There’s a darker economic reality here. Goldman Sachs analysts famously asked in a 2018 biotech report: “Is curing patients a sustainable business model?”
Heberprot-P heals diabetic wounds in weeks. The American wound care industry—dressings, negative pressure devices, hyperbaric oxygen chambers, repeated debridements, and eventually surgical amputations—generates billions in recurring revenue over months or years. A rapid cure doesn’t just fail to generate profit; it destroys an existing profitable market.
Similarly, NeuroEPO is a nasal spray that patients use at home. It requires no infusion centers, no MRI monitoring, no specialist supervision. Every element of infrastructure it eliminates is revenue that currently flows to hospitals, imaging centers, and specialty practices. The American healthcare system doesn’t just profit from expensive treatments; it has built physical infrastructure and staffing models around that expense.
A cheap, effective treatment threatens not just pharmaceutical company profits but entire healthcare delivery ecosystems.
The “Paying Twice” Phenomenon
American taxpayers already fund the foundational research that creates most new drugs. A comprehensive analysis found that NIH funding was associated with 99.4% of drugs approved by the FDA between 2010 and 2019. The NIH spent approximately $187 billion on research related to these products.
This isn’t just abstract basic science. It often includes target identification (discovering that amyloid plaques might cause Alzheimer’s), molecule discovery (creating the antibodies that eventually became Leqembi), and early clinical testing. Public money de-risks the most uncertain phases of drug development.
Then the Bayh-Dole Act of 1980 allows universities to patent these publicly-funded discoveries and license them exclusively to private companies. The company runs late-stage trials, gets FDA approval, and sets whatever price the market will bear—often selling the drug back to the government (via Medicare) and taxpayers (via insurance premiums) at monopoly rates.
The public pays to create the drug, then pays again to use it. This is the “paying twice” phenomenon.
The law technically includes “march-in rights” that allow the government to override patents if drugs aren’t made available on “reasonable terms.” In over 40 years, the NIH has never exercised this right. High prices, it has ruled, don’t constitute unreasonable terms. The safeguard is a dead letter.
The NAC Example: When Profit Can’t Be Made
To understand how this affects clinical practice, consider N-acetylcysteine (NAC). Emergency rooms use NAC to treat Tylenol overdoses—it restores glutathione and prevents liver failure. But research suggests NAC has much broader applications: reducing alcohol cravings, supporting liver recovery, treating certain psychiatric conditions.
Your doctor probably doesn’t know about these potential uses. Why? NAC is a generic supplement that costs $10 a bottle. No company will spend $100 million on clinical trials to prove new indications for a drug they can’t patent. Even if the trials proved NAC was a miracle treatment for alcohol use disorder, the company would lose money—patients would just buy the generic supplement.
So the research doesn’t get done. The FDA never updates the label. Doctors only prescribe what’s “indicated,” so they remain unaware of benefits that could help their patients. The information stays locked in academic journals that practitioners never read.
This is the “orphan generic problem,” and it affects countless potential treatments that could help patients but can’t generate profits.
Part Five: The Racial Disparity Connection
The absence of these Cuban drugs doesn’t affect all Americans equally. The populations most burdened by Alzheimer’s, lung cancer, and diabetic amputations are the same populations that face the greatest barriers to accessing expensive American treatments.
Alzheimer’s Disease
Black Americans are twice as likely to develop Alzheimer’s disease as white Americans. Hispanic Americans are 1.5 times more likely. Yet these populations were historically underrepresented in the clinical trials for drugs like Leqembi—meaning the “standard of care” was optimized for white populations.
NeuroEPO’s delivery model—a nasal spray usable at home without specialized monitoring—would specifically benefit communities with limited access to major medical centers. Its low cost would eliminate the financial barriers that keep many patients from cutting-edge treatments. But it remains unavailable.
Lung Cancer
Black men have the highest incidence rate of lung cancer in the United States. Black patients are less likely to be diagnosed at an early stage, making late-stage treatments like CIMAvax—designed specifically for advanced disease after chemotherapy—critically important for this demographic.
The checkpoint inhibitors that dominate American oncology require extensive tumor biomarker testing, regular monitoring for autoimmune complications, and access to specialized cancer centers. Rural and underserved communities often lack this infrastructure. CIMAvax’s simpler administration and milder side effect profile would make it more accessible—but American patients can’t access it.
Diabetic Amputations
The disparity here is starkest. Diabetic amputation rates among Black Americans are 2-4 times higher than among white Americans. These are often called “mega-disparities” because they dwarf nearly every other health inequity by race.
Heberprot-P directly prevents the outcome—amputation—that drives this disparity. Its deployment in Cuba has dramatically reduced amputation rates. But the regulatory and economic barriers described above keep it from reaching American patients who need it most.
There’s a painful irony here: the populations most harmed by expensive American healthcare are the ones who would most benefit from accessible Cuban alternatives.
Part Six: The Path Forward
Current Bridges
Some pathways are beginning to open. The University of Saskatchewan trial of NeuroEPO represents a potential bridge—Canadian validation of Cuban data using Western standards could eventually pressure the FDA or generate evidence that U.S. researchers can’t ignore.
Roswell Park’s ongoing CIMAvax research continues to produce promising data. If the combination with checkpoint inhibitors proves especially effective for the “non-responder” population, it could create a market niche that existing American drugs can’t fill.
Some American patients aren’t waiting. Medical tourism to Cuba has emerged as a phenomenon, with cancer patients traveling to Havana for CIMAvax and returning with doses packed in refrigerated lunch boxes. They’re technically violating U.S. law, but when you’ve been given months to live, legal technicalities feel less pressing than survival.
What Would Need to Change
Meaningful access would require several shifts:
Regulatory reform: Treating Cuban drugs like any other foreign pharmaceutical—removing the special licensing requirements that apply only to Cuba. Ensuring that OFAC licenses for clinical trials include clear pathways to commercial sale.
Research funding reform: Creating public funding mechanisms specifically to research “unprofitable” drugs—treatments that could help patients but can’t generate patent-protected returns. The NIH could fund trials for promising generic compounds that no private company will pursue.
Pricing reform: Enforcing the “reasonable terms” provisions of the Bayh-Dole Act, or creating new mechanisms to ensure that publicly-funded discoveries result in publicly-accessible treatments.
Patent reform: Reconsidering whether the 20-year monopoly model is the best way to incentivize innovation, or whether alternative approaches (prizes, government purchases, compulsory licensing) might produce better public health outcomes.
None of these changes are imminent. The pharmaceutical industry is among the most powerful lobbying forces in Washington. The Cuba embargo has survived 11 presidents and shows no signs of ending. The structures that produce expensive, modestly-effective drugs while blocking cheap, promising alternatives are deeply entrenched.
Part Seven: What This Means for Patients and Families
If you or a family member is facing Alzheimer’s, lung cancer, or diabetic complications, the existence of these Cuban treatments is simultaneously hopeful and frustrating.
For Alzheimer’s Patients
The University of Saskatchewan trial is currently recruiting patients. If you’re in Canada or can travel there, this may be worth exploring. In the U.S., no legal pathway currently exists. The best approach is to monitor research developments and advocate for policy changes that would allow clinical trials to proceed.
For Lung Cancer Patients
Ask your oncologist about the Roswell Park clinical trials for CIMAvax. Not everyone qualifies, but it’s worth exploring. For those considering travel to Cuba, understand the legal risks and consult with an attorney familiar with OFAC regulations.
For Diabetic Patients with Foot Ulcers
Heberprot-P is available in Cuba and several other countries through medical tourism channels. Specialized medical tourism services can facilitate treatment in Havana. This is legally complicated for Americans, but some patients have pursued it when facing imminent amputation.
For Everyone
Advocate. The embargo is not a natural law—it’s a policy choice that can be changed. Organizations like the Washington Office on Latin America and the US-Cuba Normalization Coalition work on these issues. Contact your congressional representatives. Support research funding for “unprofitable” treatments. Push for pharmaceutical pricing reform.
The researchers in Havana didn’t develop these treatments to make a political point. They developed them because people were sick and they wanted to help. Whether American patients can benefit from that work remains a political question—one that voters and advocates can influence.
What Innovation Actually Means
The United States leads the world in pharmaceutical innovation by certain metrics. We spend the most on research. We produce the most patents. We generate the highest revenues. Our drug companies are among the most valuable corporations on earth.
But innovation should mean more than shareholder returns. It should mean better outcomes for patients. And by that measure, something has gone profoundly wrong.
A small island nation under economic embargo—with a fraction of our resources, cut off from global capital markets and advanced technology—has developed treatments that may work better than ours for some of the most devastating conditions humans face. They did it by orienting their research around public health outcomes rather than profit maximization. They designed for accessibility rather than market exclusivity. They optimized for safety and ease of use rather than infrastructure-dependent complexity.
This doesn’t mean Cuba’s healthcare system is superior overall—it faces enormous challenges, many created by the embargo itself. But in these specific cases, the “closed cycle” model of public research producing public goods has generated innovations that the “blockbuster” model of private research producing private profits has not.
The uncomfortable question is what we do with that knowledge. We can continue as we are: funding research publicly, privatizing the results, paying twice, and accepting that effective treatments may remain forever out of reach because they’re not profitable enough. Or we can demand a system that measures success by whether patients get better, not by whether shareholders get richer.
Ninety miles is not very far. Sixty years is a very long time. But the distance between effective medicine and American patients isn’t ultimately measured in geography or history. It’s measured in policy choices—choices we can change whenever we decide that patients matter more than profits.
Joel Blackstock, LICSW-S, is the Clinical Director of Taproot Therapy Collective in Birmingham, Alabama, where he specializes in complex trauma treatment using qEEG brain mapping, Brainspotting, and Emotional Transformation Therapy.
References
NeuroEPO and Alzheimer’s Disease
- NeuroEPO plus (NeuralCIM®) in mild-to-moderate Alzheimer’s clinical syndrome: the ATHENEA randomized clinical trial – PubMed Central
- Evaluation of the Safety and Efficacy of NeuroEPO in Subjects With Mild to Moderate Alzheimer’s Disease – ClinicalTrials.gov
- Little-Known Cuban Alzheimer’s Drug Goes to Human Trials in Canada – Being Patient
- Support needed for Phase 2 trials of life-changing Alzheimer’s treatment – University of Saskatchewan
- 2nd clinical trials for promising Alzheimer’s drug to take place at U of Sask – CBC News
Leqembi and U.S. Alzheimer’s Treatments
- Early Alzheimer’s Patients Continue to Benefit from Four Years of LEQEMBI Therapy – Eisai
- EISAI’S APPROACH TO U.S. PRICING FOR LEQEMBI – Eisai Media
- The efficacy and safety of lecanemab 10 mg/kg biweekly compared to a placebo – PubMed Central
- Aducanumab – Wikipedia
- Leqembi – AlzForum
CIMAvax and Lung Cancer
- CimaVax-EGF – Wikipedia
- CIMAvax Lung Cancer Vaccine – Roswell Park Comprehensive Cancer Center
- A Phase III Clinical Trial of the Epidermal Growth Factor Vaccine CIMAvax-EGF – AACR Journals
- Final results from a phase II trial of CIMAvax-EGF and nivolumab – ASCO Publications
- This Cuban lung cancer drug is giving some U.S. patients hope – PBS NewsHour
- Cuba Has a Lung Cancer Vaccine. But American Patients Can’t Get It Without Breaking the Law – Pulitzer Center
Heberprot-P and Diabetic Foot Ulcers
- Heberprot-P: a novel product for treating advanced diabetic foot ulcer – PubMed
- UA Health Sciences Researchers Look to Collaborate With Cuba on Diabetic Wound Therapy – University of Arizona
- Heberprot-P, a revolutionary treatment brings hope – Granma
- US–Cuba Health Cooperation: Time to Leverage Easier Access to Cuban Health Innovations – SciELO Public Health
- Treatment for Diabetic Foot Ulcer Utilizing Heberprot-P – CubaHeal
- Cuba Blockade Hurts US Patients Too – Consortium News
The Embargo and Trade Policy
- United States embargo against Cuba – Wikipedia
- Cuba Embargoed: U.S. Trade Sanctions Turn Sixty – National Security Archive
- Today marks 60 years of the Cuban embargo. What exactly is it? – Responsible Statecraft
- Understanding the Failure of the U.S. Embargo on Cuba – Washington Office on Latin America
- 50 Years of U.S. Embargo: Cuba’s Health Consequences and Lessons – PLOS Medicine
Pharmaceutical Economics and the “Paying Twice” Problem
- Who Develops Medicines? An Analysis of NIH Grants – Vital Transformation
- Comparison of Research Spending on New Drug Approvals by the US National Institutes of Health vs the Pharmaceutical Industry – PubMed Central
- The Bayh-Dole Act: Selected Issues in Patent Policy – Congressional Research Service
- The Bayh-Dole Act and the Debate Over “Reasonable Price” March-In Rights – Federalist Society
- Public Dollars, Private Profits? Cancer Drug’s Cost Sky High Despite Taxpayer Funding – Oncology News Central
- Dunking on the Pharma Industry: An In-Depth Analysis of Mark Cuban’s War on Drug Prices – Drug Patent Watch
Cuban Biotechnology
- Cuban Medicine and Biotechnology – History of Science in Latin America and the Caribbean
- Biotechnology Development in Cuba: Challenges in the Economic Strategy Beyond 2021 – Columbia Law School
- Cuba—U.S. scientific collaboration: Beyond the embargo – PLOS ONE



















0 Comments